Nosocomial infections cause significant morbidity and mortality within the United States each year. A recent article in the Journal of the American Medical Association surveyed 20% of hospitals in the United Stats and found that hospital infections cause patients on average to stay 9.58 more days in the hospital, spend $38,656 more on their hospitalization, and increase their risk of dying by 4.31%. 1 In response to this nationwide problem, the Join Commission on Accreditation of Hospital Organizations (JCAHO) has approved revised standards for 2004, which seek to help prevent the occurrence of health care-associated infections.2
The American Hospital Association has estimated that there are 965,962 staffed, registered hospital beds within the United States.3 A handheld device, which is used to call the nurse and/or manipulate a television within the hospital room, is found in the majority of hospital beds within the United States. These handheld devices are more formally referred to as Nurse Call Pillow Speakers, but we will henceforth refer to them as "handsets" or "units." All of the units consist of formed plastic anterior and posterior pieces, which surround electrical components, including a microphone, a speaker, and buttons to control both nuse call and the function of the television. Numerous cracks and crevices were noted on the units that could allow the ingress or egress of microbes, spores, and/or bodily fluids.
In late 2002, the authors were approached by members of the biomedical staff of a suburban hospital. They had for some time been concerned about the cleaning, repair, and replacement of such handsets that was felt to be due to the accretion of biologic material within the handsets. However, they had more recently become concerned about the possible impact of this material on the incidence of nosocomial infections. Please see Figs 1 and 2 for visual representations of the biologic material that was found within the handsets.
 Fig.1
Fig.1 Fig.2
Fig.2
Tyco International is a prominent distributor of nurse call systems and accessories through its subsidiary, Tyco Healthcare; representatives of the company have estimated to the authors that 50% to 60% of all hospital beds within the United States have a handset that is substantially similar to the handsets used by this suburban hospital (Personal communication: Daryle Longstreth, Manager of Independent Distribution, Tyco Healthcare, Tyco International Ltd., 15 Hampshire Street, Mansfield, MA 02048). Thus, over 500,000 of these units may be utilized daily throughout the United States. The authors carefully observed the use of the hospital handsets by patients and were impressed by the frequency of hand contact and the proximity of the handset to the mouth and nose of the patient because of the microphone and speaker present within the unit.
The authors set out to determine the present body of research dealing with such handsets. After initial searches were unable to find references to the microbial flora of the handsets, the library staff of the Texas Tech University Medical Center was consulted. Again, no direct reference to the study of the microbial contamination of such handsets was found.
The authors were able to find numerous studies that evaluated inanimate items within the local hospital environment for their potential to both harbor and transmit infection. In 1978, Bagshawe et al reviewed prior studies of the hospital environment and found that bedding, x-ray cassettes, stethoscopes, sphygmomanometers, baths and bowls, shaving brushes, crockery, bedpans, urinals, respiratory apparatus, and toys had the ability to transmit infection; however, they noted that "only respiratory apparatus and baths have been established as consistent reservoirs from which infection spreads easily and widely."4 More recent studies have evaluated "bedsheets, floor and bedside chairs"5 and hospital mattresses.6 After careful consideration, the authors decided to pursue a pilot study to determine the microbial contamination of hospital bed handsets.
Materials and Methods
The present study was carried out in 2003 in a suburban Texas hospital with 410 beds. A handset designed to facilitate nurse communication and television control was found in the beds of 370 rooms (90.2%). These handsets were manufactured by Curbell of Orchard Park, NY, and were classified as generation 2 (Gen2) or generation 3 (Gen3) series handsets. The authors' intent to study the microbial flora of these units was communicated to the local institutional review board, and consent was given to evaluate the units. These units were found in all wards of the hospital and within the adult and pediatric intensive care units. Using a computer randomization program, 115 units were randomly chosen for study, representing 31% of the available units. In an effort to maximize confidentiality, each of the chosen handsets was assigned a unique identifier. The standard cleaning regimen of this hospital was to wipe the outside of the handset with a disinfectant liquid between each patient stay. Immediately after the dismissal of a patient and after standard cleansing of the units in preparation for the next patient, the handsets were transported in a sterile fashion to the biomedical department for study. The units were opened using sterile technique and cultured on a daily basis within 24 hours of removal from the hospital room. Sterile gloves and a sterile container were used to transport the units to the research area. At the time of investigation, the units were opened in a sterile fashion, and a sterile swab was used to collect microorganisms and biologic debris from the interior of the handset. Separate cultures were
obtained from both the front and the back of each unit. The swabs were used to inoculate sheep red blood agar and chocolate agar plates. These plates were marked with the unique identifier that had been assigned to the hospital handset. The hospital microbiologists reported the culture results for each unique identifier, and the results were stored and tabulated in a computer spreadsheet. At the conclusion of the study, the key linking the unique identifier to the original hospital room of the unit was destroyed to ensure complete confidentiality of the data in accordance with the Health Insurance Portability and Accountability Act of 1996 (HIPPA). After the units were cultured, they were cleansed thoroughly prior to being reassembled, using the hospital disinfectant solution that was routinely used on the exterior of the units. The units were then tested for functionality; fully functioning units were returned to service, and all dysfunctional units were replaced with new units per the usual hospital protocol.
Results
The cultures of 12 units (10.4%) revealed no microorganisms. One hundred three units (89.6%) had cultures that grew microorganisms. Of the handsets that were found to contain microorganisms, 48 units (46.6%) had only 1 microorganism, and 55 units (53.4%) had multiple organisms, including 33 units (32.0%) with 2 microorganisms, 21 units (20.4%) with 3 microorganisms, and 1 unit (1.0%) with 4 microorganisms.
The microorganisms identified included 90 isolates (87.4%) of coagulase-negative staphylococcus, 51 isolates (49.5%) of bacillus species, 13 isolates (12.6%) off ungal species, 8 isolates (7.8%) of nonhemolytic streptococcus species, 7 isolates (6.8%) of a-hemolytic streptococcus species, 1 isolate (1.0%) of methicillin-sensitive Staphylococcus aureus, and 1 isolate (1.0%) of methicillin-resistant Staphylococcus aureus. It was also noted that 101 of the handsets contained either coagulase-negative staphylococcus or bacillus species, representing 87.8% of all units and 98.1% of the culture-positive units.
Discussion
This pilot study of the microbial contamination of hospital bed handsets found a high degree of contamination, including significant numbers of bacteria that are known to be the etiologic agent of nosocomial infections. The 2 most common organisms, coagulase-negative staphylococcus and bacillus species, are considered normal flora of the skin. Because the handsets have, by design, frequent contact with the skin, such contamination was expected. However, the high degree of such contamination, 87.8% of all units and 98.1% of culture-positive units, does exhibit the potential of the units for serving asa reservoir for microbes encountered on the skin surface. Further study should include the antibiotic sensitivity of the coagulase-negative staphylococcus because the incidence of methicillin-resistant coagulase-negative staphylococcus is being followed by the National Nosocomial Infections Surveillance (NNIS) System. 7
Fungal species were identified in 12.6% of the colonized handsets. Further study should elucidate the specific fungi found within the units. Many species could be problematic in a reservoir located near relatively immunocompromised patients. Candida species on the hands of health care workers have been shown to be transmitted to patients in whom they have been implicated in nosocomial infections.8 In addition, aspergillus colonies have been shown to release spores that may remain airborne for prolonged periods and infect subjects both directly and through nasopharyngeal colonization.9 Thus, fungal colonies within the handsets could infect patients through either direct contact with the unit or inhalation of spores released from the reservoir.
Nonhemolytic streptococcus species were found in 7.8% of the colonized handsets. Further study should include the specific identification of these colonies to assess the incidence of enterococcus species and the antimicrobial sensitivity of these isolates. Enterococcus is known to readily colonize such materials, and vancomycin-resistant enterococcus (VRE) is responsible for an increasing number of severe nosocomial infections.10
Alpha hemolytic streptococcus species were found in 6.8% of the colonized handsets. Again, further study should elucidate the specific bacteria involved.
Staphylococcus aureus species were identified in 2 handsets, and one of these isolates was identified as methicillin-resistant (MRSA). The authors feel that the importance of this finding, although a small percentage of the total handsets, should not be underestimated. A review article concerning the control of MRSA in the hospital setting concluded that aggressive control efforts are justified; institutions with an elevated prevalence of MRSAwere instructed to eradicatecarefullythe possible reservoirs of this organism.11 Our study results suggest that handsets can function as a reservoir for MRSA.
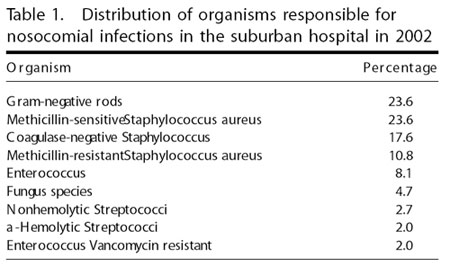
Table 1 presents the distribution of organisms responsible for nosocomial infections documented at the suburban hospital in the year 2002. It is notable that gram-negative rods were responsible for 23.6% of all nosocomial infections, which tied with methicillin-resistant staphylococcus for the most common cause of nosocomial infection within the hospital. Conversely, no gram-negative rods were found in the isolates from the handsets. Sanderson and Alshafi studied the environmental contamination by organisms causing urinary tract infection and published their findings in 1995 in the Journal of Hospital Infection.5 They cited studies in which gram-positive bacteria may be able to survive several weeks in dry conditions,12 whereas gram-negative bacteria were able to survive "some hours" or longer, if biologic material for nutrients was available.13 Although the biologic material appears to the authors to have entered the units in a liquid form, it was uniformly dessicated when cultured for this study. It seems that gram-negative bacteria could have been present in the units until the water in the fluid evaporated or was catabolized. The delay between the cessation of contact with the patient and the culturing of the inside of the units may have significantly decreased the probability of culturing gram-negative bacteria from the units. Gram's stains of the biologic material within the units may provide an answer to this question and should be considered on further study.
The authors were intrigued by the amount of biologic material, visible to the naked eye, within the handsets. It is doubtful that this mass of material could be transferred from the surface of the skin. Again, the entrance of body fluids into the units appears to be the most likely occurrence. Although the appearance of the material was suggestive of dried urine or blood, further testing would be required to elucidate with certainty the etiology of the material.
The authors believe that the present rate of contamination, distribution of microorganisms, and degree of patient contact in hospital handsets uniquely positions them to serve as a source of nosocomial infections. However, it should be noted that no evidence currently exists that conclusively links the contamination of hospital handsets with nosocomial infections.
Present recommendations for the decontamination of hospital equipment include the cleaning of the item, followed by disinfection; cleaning is a process in which the microorganisms and the organic material on which they thrive are removed, whereas disinfection is the treatment of equipment to reduce the level of microorganisms to a level that is not harmful to human health.14 The standard method of decontaminating the units, currently utilized, was obviously inadequate. The external case was both cleaned and disinfected, but the interior of the units was able to serve asa reservoir for microorganisms.
It is assumed that the present contamination could be relieved by one of the following methods: disposing of the units after each patient contact, cleaning and disinfecting the interior of the units after each patient contact, or the application of a clean, single-use external barrier to the unit. Although disposal of the units after each patient contact would provide the most effective means ofi nfection control, the average price of a unit ranges from $40.95 to $116.95, and such disposal would add significantly to the cost of health care (Personal communication: Daryle Longstreth, Manager of Independent Distribution, Tyco Healthcare, Tyco International Ltd., 15 Hampshire Street, Mansfield, MA 02048). Cleaning and disinfection of the interior of the units would reduce the potential for infection, but this would require an increase in staff personnel hours because the units are not presently designed for internal cleaning. In addition, the handsets are electronic devices; the exposure of the electronic components to cleaning and disinfecting solutions or chemicals may have a deleterious effect on the functioning of the devices. This exposure may result in a shortened lifespan for the units. Clean, disposable covers for the handsets are presently available for $1.78 each (Personal communication: Daryle Longstreth). Further study should be conducted to evaluate the relationship between the organisms found on these handsets and the occurrence of nosocomial infections. In addition, studies should be conducted concerning the effectiveness and relative cost of the above methods of intervention.
The authors thank Abbott Laboratories and their pharmaceutical representative, David Bowren, for the preliminary grant for this research.
